\(\Delta H\) is the enthalpy change (in kJ or kJ mol1) c is the specific heat capacity of water It is a constant, 418 kJ kg 1 ˚C 1 and is found in the data bookThe equation for the standard heat of formation of B 2 H 6 is 2B(s) 3H 2 (g) > B 2 H 6 (g) DeltaH = unknown I am assuming the other equations you have written are correct You just need to manipulate these equations to get the desired equation above just as you startedδ (delta) convention, for designating allenes and other cumulated dienes in rings named according to a noncumulated parent structure;
/GettyImages-175519466-589b85f83df78c4758997c9e.jpg)
Greek Alphabet And Symbols In Chemistry
Delta s meaning chemistry
Delta s meaning chemistry-Delta definition is the 4th letter of the Greek alphabet How to use delta in a sentenceUppercase delta (Δ) often means "change" or "the change in" in mathematics For example, if the variable "x" stands for the movement of an object, then "Δx" means "the change in movement" Scientists use this mathematical meaning of delta often in physics, chemistry, and engineering, and it appears often in word problems



Gibbs Free Energy And Spontaneity Video Khan Academy
Greek δέλτα délta, ) is the fourth letter of the Greek alphabetIn the system of Greek numerals it has a value of 4 It was derived from the Phoenician letter dalet 𐤃, Letters that come from delta include Latin D and Cyrillic Д A river delta (originally, the Nile River delta) is so named because its shape approximatesThe equation of delta t is ΔT = T2 T1 To the left there is the drawing of a tubular heat exchanger There is the cooling water entering at point B and leaving warmer at point D And there is the liquid stream to be cooled, entering point C and getting out at point A To get a usable ΔT, one should compare the same liquid going in and outTips on understanding the difference between delta H and delta S Let's decide what delta H and delta S are Delta H is enthalpy And the reason I made my H capitalised, is because that's how I remember that delta H is enthalpy It has an H in it This measurement of heat or energy transfer
Equilibria, ∆G, ∆H and ∆S In a wide range of situations, we will see that understanding ∆G, ∆H and ∆S can help us understand where an equilibrium lies and often allow us to control whether the reactants or products are favored For some of you, a little algebra might be helpful (Or just skip to "What it means")Crystal field splitting energy (Δ) for metal complexes;Translingual ·(mathematics, sciences) Alternative form of ∆ change in a variable· (chemistry) Used on the reaction arrow in a chemical equation, to show that energy in the form of heat is added to the reaction· (mathematics, set theory) Used to represent the symmetric difference (also known as the disjunctive union) of two sets (genetics) Used
Define delta delta synonyms, delta pronunciation, delta translation, English dictionary definition of delta An area of the southcentral United States extending on either side of the Mississippi River from Memphis, Tennessee, to Vicksburg, Mississippi adj Chemistry 1This article is a summary of common equations and quantities in thermodynamics (see thermodynamic equations for more elaboration) SI units are used for absolute temperature, not Celsius or FahrenheitLisa asked in Science & Mathematics Chemistry · 8 years ago what does delta S mean for a reaction?



Difference Between Enthalpy And Entropy With Its Practical Applications In Real Life



Gibbs Free Energy And Spontaneity Video Khan Academy
Delta H refers to a change in enthalpy, and the degree symbol signifies that the enthalpy change is for standard conditions The standard state of a substance is the state that is most stable at K (room temperature) and 1 atm of pressureS = k ln W In this equation, S is the entropy of the system, k is a proportionality constant equal to the ideal gas constant divided by Avogadro's constant, ln represents a logarithm to the base e, and W is the number of equivalent ways of describing the state of the system According to this equation, the entropy of a system increases as theLatex\Delta U = Q W/latex In this equation, U is the total energy of the system, Q is heat, and W is work In chemical systems, the most common type of work is pressurevolume (PV) work, in which the volume of a gas changes Substituting this in for work in the above equation, we can define the change in internal energy for a chemical system


Xenon Definition Properties Atomic Mass Compounds Facts Britannica


6 2 3 3 The Arrhenius Law Activation Energies Chemistry Libretexts
In chemistry delta (Δ) is used for the symbol of heat You must have seen this symbol specially in many chemical reactions which means the reaction takes place in presence of heat FYI in physics it means change 28K viewsWhen delta G > 0 It's a nonspontaneous reaction When delta G < 0 It's a spontaneousThe equation for the standard heat of formation of B 2 H 6 is 2B(s) 3H 2 (g) > B 2 H 6 (g) DeltaH = unknown I am assuming the other equations you have written are correct You just need to manipulate these equations to get the desired equation above just as you started



Chemistry 2e Open Textbook Library
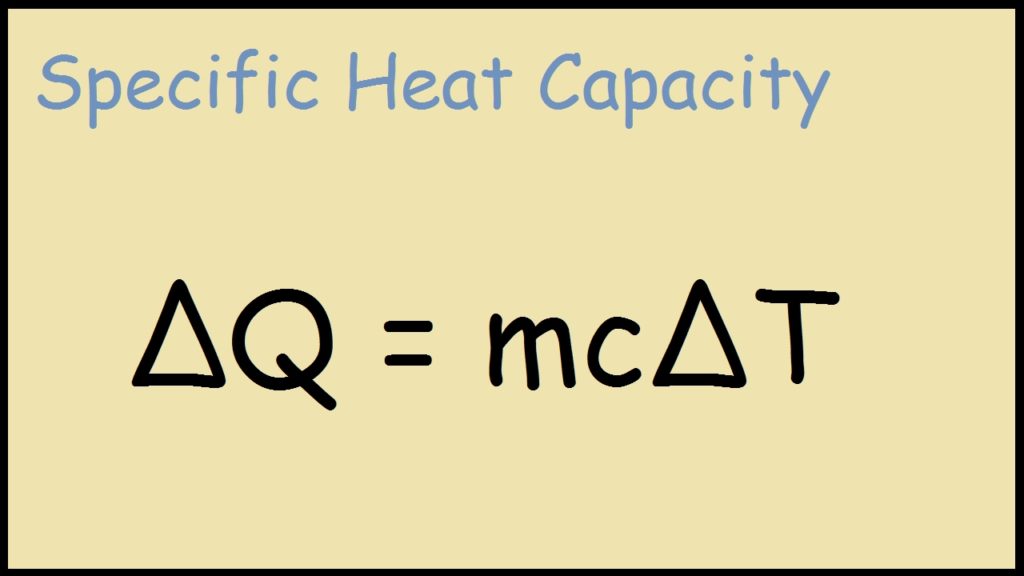


Specific Heat Formula Definition Equations Examples
Delta G = Delta H T Delta S Chad explains the relationship between Gibbs Free Energy, Enthalpy and Entropy and when a reaction will be spontaneousDelta / ˈ d ɛ l t ə / (uppercase Δ, lowercase δ or 𝛿;Enthalpy of Atomization Definition (Chemistry) Enthalpy Change Definition in Science Calculate Energy Required to Turn Ice Into Steam Coffee Cup and Bomb Calorimetry Heat of Formation Worked Problem Calorimeter Definition in Chemistry Heat of Formation Table for Common Compounds



Entropy Of Surrounding Chemistry Thermodynamics Meritnation Com



15 2 Predict The Entropy Change For A Given Reaction Or Process Hl Ib Chemistry Youtube
Delta definition is the 4th letter of the Greek alphabet How to use delta in a sentenceEquilibria, ∆G, ∆H and ∆S In a wide range of situations, we will see that understanding ∆G, ∆H and ∆S can help us understand where an equilibrium lies and often allow us to control whether the reactants or products are favored For some of you, a little algebra might be helpful (Or just skip to "What it means")Adam v asked in Science & Mathematics Chemistry · 1 decade ago What is delta S for the vaporization of water?


Gibbs Free Energy


Chemical Thermodynamics
The letter S in chemistry is used in thermodynamics It stands for entropy For example, the change in the entropy of the universe (delta S) is equal to delta S of the system plus delta S of theDelta G = Delta H T Delta S Chad explains the relationship between Gibbs Free Energy, Enthalpy and Entropy and when a reaction will be spontaneousDelta G is the symbol for spontaneity, and there are two factors which can affect it, enthalpy and entropy Enthalpy the heat content of a system at constant pressure Entropy the amount of disorder in the system Below is a table to summarize it up!



5 1 Delta Hf And Delta Hc Calculations Sl Ib Chemistry Youtube


What Does The Symbol D Delta Mean In Chemistry Quora
In chemistry, entropy is represented by the capital letter S, and it is a thermodynamic function that describes the randomness and disorder of molecules based on the number of differentConsider first an endothermic reaction (positive \(\Delta H\)) that also displays an increase in entropy (positive \(\Delta S\)) It is the entropy term that favors the reaction Therefore, as the temperature increases, the \(T \Delta S\) term in the Gibbs free energy equation will begin to predominate and \(\Delta G\) will become negativeIn this case, #Delta# still means heat, but for this photochemicallycatalyzed reaction, you could have also used UV light, which was indicated by #hnu# since #E = hnu# tends to be used to evaluate the energy of a photon (or a #"mol"# of photons)



Solved 14 When The Delta Sign A Appears Over The React Chegg Com


A Level A Level 1 1 Advanced Introduction To Enthalpy Energy Changes Reaction Combustion Formation In Chemical Reactions Ks5 Gce Chemistry Revision Notes
In this case, #Delta# still means heat, but for this photochemicallycatalyzed reaction, you could have also used UV light, which was indicated by #hnu# since #E = hnu# tends to be used to evaluate the energy of a photon (or a #"mol"# of photons)So delta S is the measure of randomness or chaos or movement, as in the particles or compounds H is the measurement of how much energy it contains within it And we can't measure H by itselfConsider first an endothermic reaction (positive \(\Delta H\)) that also displays an increase in entropy (positive \(\Delta S\)) It is the entropy term that favors the reaction Therefore, as the temperature increases, the \(T \Delta S\) term in the Gibbs free energy equation will begin to predominate and \(\Delta G\) will become negative



Enthalpy
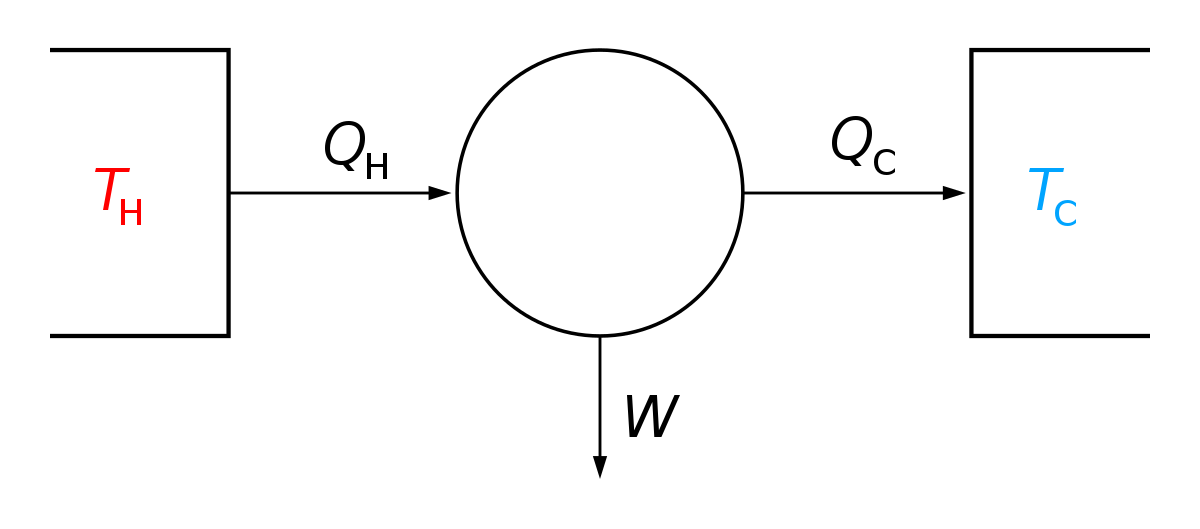


Gibbs Free Energy Wikipedia
Calculate delta S degree rxn for this balanced chemical equation 2NO(g)O2(g)>2NO2(g) Express your answer to one decimal place and include the appropriate units In order for a process to beWhere G is Gibb's free energyThe normal boiling point of water is 100C and deltaHvap is 440kJmol1



19 7 Dg And K As Functions Of Temperature Chemistry Libretexts



D Wiktionary
'The forests and estuaries of the delta also benefited from huge floods released into the river from behind dams during the El Niño years of the 1980s and 1990s' 'Water flow in the rivers decreased when the region's climate changed about 5,000 years ago and wind began to winnow the river delta's dried sediments'Delta S equals zero when the reaction is reversible because entropy is a state function When the process is reversible, it starts and ends in the same place making entropy equal to zeroThe second law states that there exists a useful state variable called entropy The change in entropy (delta S) is equal to the heat transfer (delta Q) divided by the temperature (T) delta S = (delta q) / T For a given physical process, the entropy of the system and the environment will remain a constant if the process can be reversed



Delta Definition And Meaning Collins English Dictionary


Chemical Reactivity
Delta S refers to the change of Entropy And delta H refers to the change of enthalpy Entropy is the variable that becomes constant in an adiabatic process The following equation relates the change of Entropy with the change of enthalpy Delta G = Delta H (Delta S)×Temperature;DELTA E, DELTA H, DELTA T WHAT DOES IT MEAN?3 Delta H (ΔH) The difference between two colors in the threedimensional L*a*b* color space is known as delta E However, this distance is only partly suitable for evaluating measured gray balance The current ISO norm includes the hue difference delta H for primary colors and grayscalesIllustrated Glossary of Organic Chemistry δ A symbol which indicates that an atom or region with a deficiency of electron density, often because of resonance delocalization, electronegativity differences, or inductive effects



Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Practice Problems Youtube



3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
In a chemistry formula for reactions, you could insert a delta meaning the addition of heat In particle physics, delta symbol represents the delta particles while in quantum mechanics it denotes uncertainty in physical variables While in law the delta letter is used to represent the defendant, it is used to denote deletion of a gene in geneticsChemistry Δ (delta) vs Λ (lambda) isomers to indicate right vs left handed propeller twists in coordination complexes;Delta H is when a reaction is favorable T is in Kelvin and will always be positive delta S is when it is favorable (which means that ( T x delta S) will be negative) delta G = delta H T delta S Since a negative delta H is favorable and a positive delta S is favorable, that means that a negative delta G must also be favorable



3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow



What Is The Difference Between The Usage Of D Delta Small Delta And The Partial Derivative Symbol In Thermodynamics
Say you have a reaction between solid zinc and aqueous copper sulfate We used an electrochemical cell to measure the cell potential of this reaction in non standard conditions Then I calculated the delta S value to be 06 J/mol KSigma, σ, and delta, δ, chemical shift scales IUPAC has restated their recommendation that NMR researchers use the δ chemical shift scale 2 Hopefully, this recommendation will be followed more closely in the future, since even minorThe second law states that there exists a useful state variable called entropy The change in entropy (delta S) is equal to the heat transfer (delta Q) divided by the temperature (T) delta S = (delta q) / T For a given physical process, the entropy of the system and the environment will remain a constant if the process can be reversed
/GettyImages-175519466-589b85f83df78c4758997c9e.jpg)


Greek Alphabet And Symbols In Chemistry



Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Practice Problems Youtube
Delta H is when a reaction is favorable T is in Kelvin and will always be positive delta S is when it is favorable (which means that ( T x delta S) will be negative) delta G = delta H T delta S Since a negative delta H is favorable and a positive delta S is favorable, that means that a negative delta G must also be favorableIn a chemistry formula for reactions, you could insert a delta meaning the addition of heat In particle physics, delta symbol represents the delta particles while in quantum mechanics it denotes uncertainty in physical variables While in law the delta letter is used to represent the defendant, it is used to denote deletion of a gene in geneticsUltimately, however, while all of these changes can be described in terms of Delta E, that does not mean all of them are reflected in ASTM's lightfastness rating even though they can impact how a pigment is used So, for example, returning to our earlier example, while Ultramarine Blue and Cadmiums are all given a Lightfastness rating of I by


Chemical Thermodynamics


Entropy And The Second Law Of Thermodynamics
Delta S In the world of chemistry, S is the symbol for entropy Also, in case you didn't know this, scientists use the "delta" symbol to denote a change in a quantity It follows from this that Delta S is the change in the entropy of a given system and surroundings Spontaneous processesSigma, σ, and delta, δ, chemical shift scales IUPAC has restated their recommendation that NMR researchers use the δ chemical shift scale 2 Hopefully, this recommendation will be followed more closely in the future, since even minorDefinition of delta (Entry 2 of 5) chemistry fourth in position in the structure of an organic (see organic entry 1 sense 1b (2)) molecule from a particular group or atom — symbol ƍ



Solved Use Delta H Circ And Delta S Circ



Consider The Reaction Caco3 S Cao S Clutch Prep



What Are Chemical Equations Detailed Explanation Examples


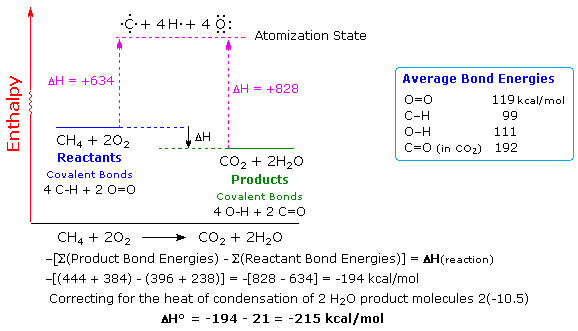
Potential Kinetic Free And Activation Energy Boundless Biology



Endergonic Reaction Wikipedia


Gibbs Free Energy Changes Equation Calculations Reaction Feasibility Extraction Of Metals Cell Emf Gce A Level Chemistry Revision Notes



Classify The Following Phase Changes By Th Clutch Prep



Gibbs Free Energy Change Dg And Entropy Change Ds Secondary Science 4 All


What Is Enthalpy Definition Endothermic Exothermic Reaction



Understanding The Difference Between Delta H And Delta S Concept Chemistry Video By Brightstorm
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)


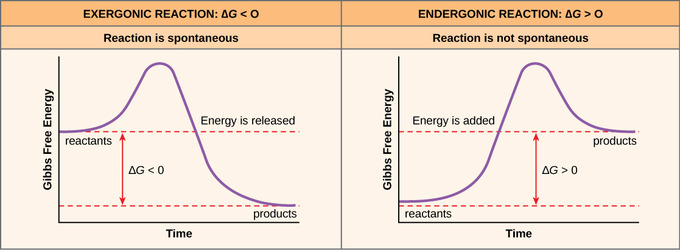
Endergonic Vs Exergonic Reactions And Processes



Calculating Internal Energy Delta E Of A Chemical Reaction Explained Youtube


Aqa A Level Chemistry Unit 5 Section 3 5 1 Thermodynamics Free Energy Change G And Entropy Change S



Second Law Of Thermodynamics
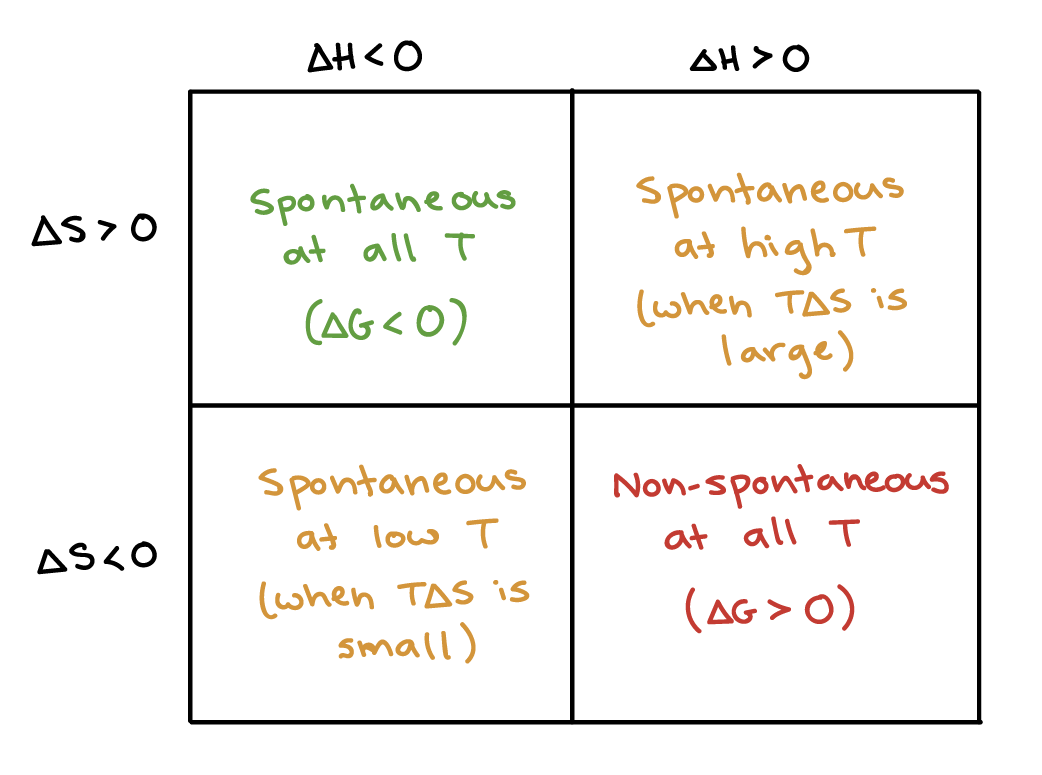


Gibbs Free Energy And Spontaneity Article Khan Academy



Energy Changes In Chemical Reactions Energy And Chemical Change Siyavula
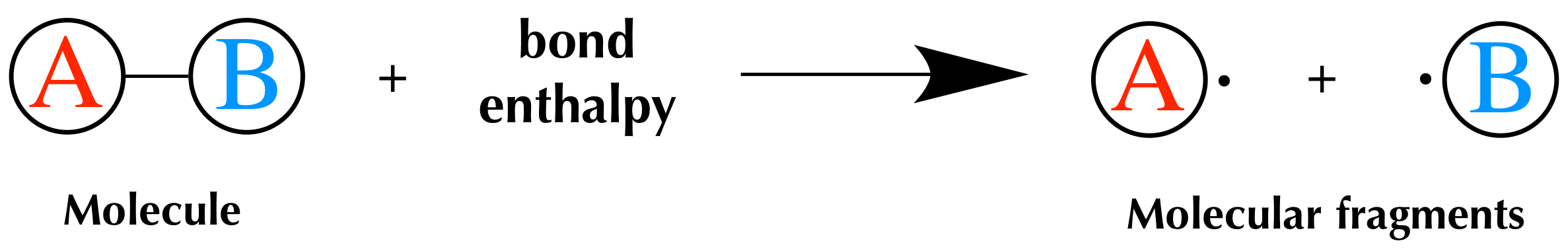


Bond Enthalpy And Enthalpy Of Reaction Article Khan Academy


Gibbs Free Energy Changes Equation Calculations Reaction Feasibility Extraction Of Metals Cell Emf Gce A Level Chemistry Revision Notes
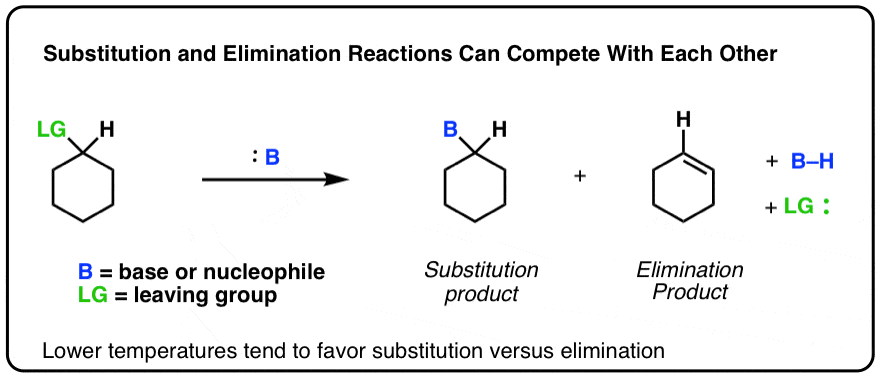


Elimination Reactions Are Favored By Heat Master Organic Chemistry
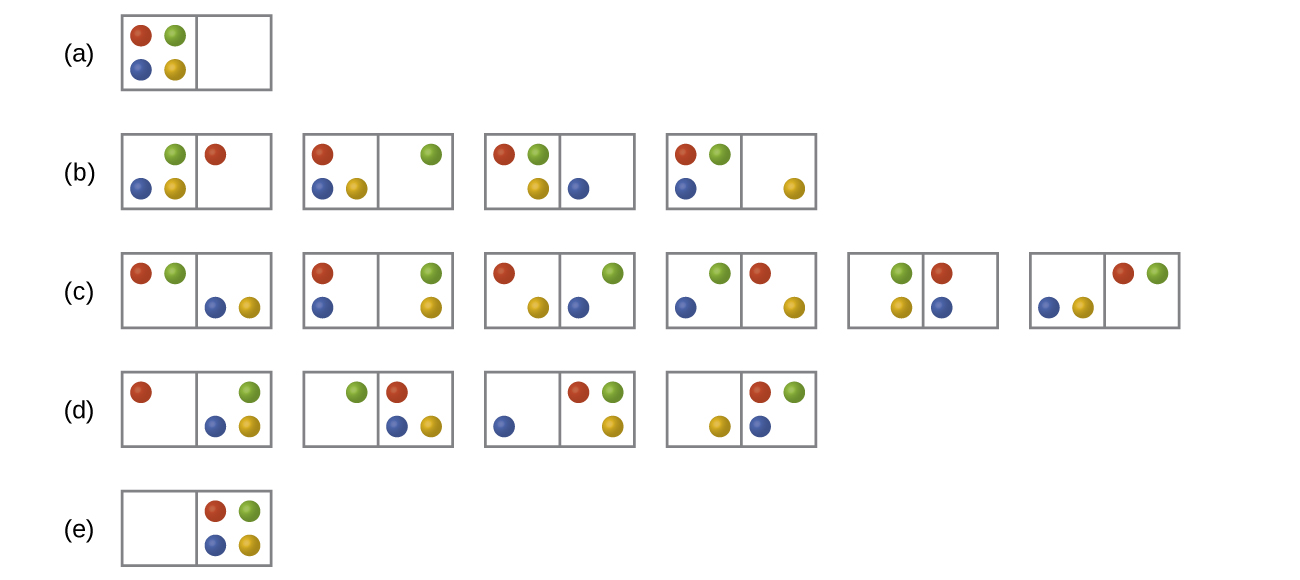


16 2 Entropy Chemistry
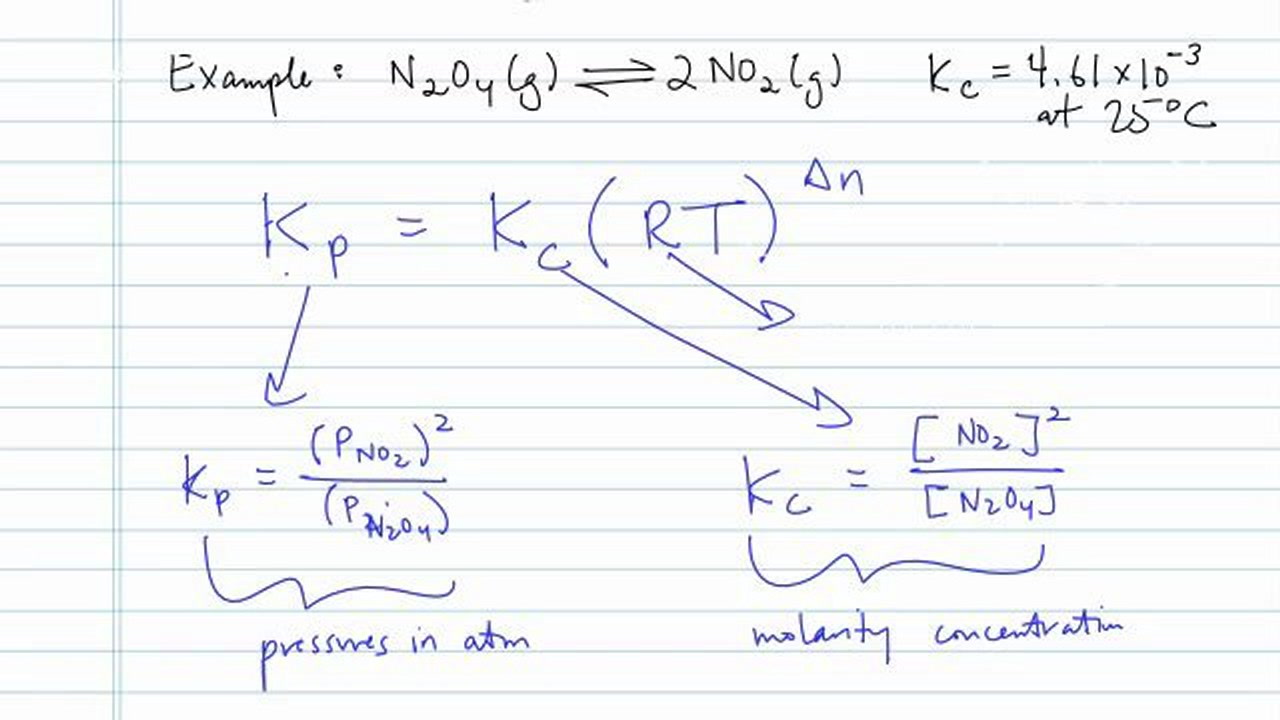


Tips For Converting To Kp From Kc Concept Chemistry Video By Brightstorm


Gibbs Free Energy Wikipedia
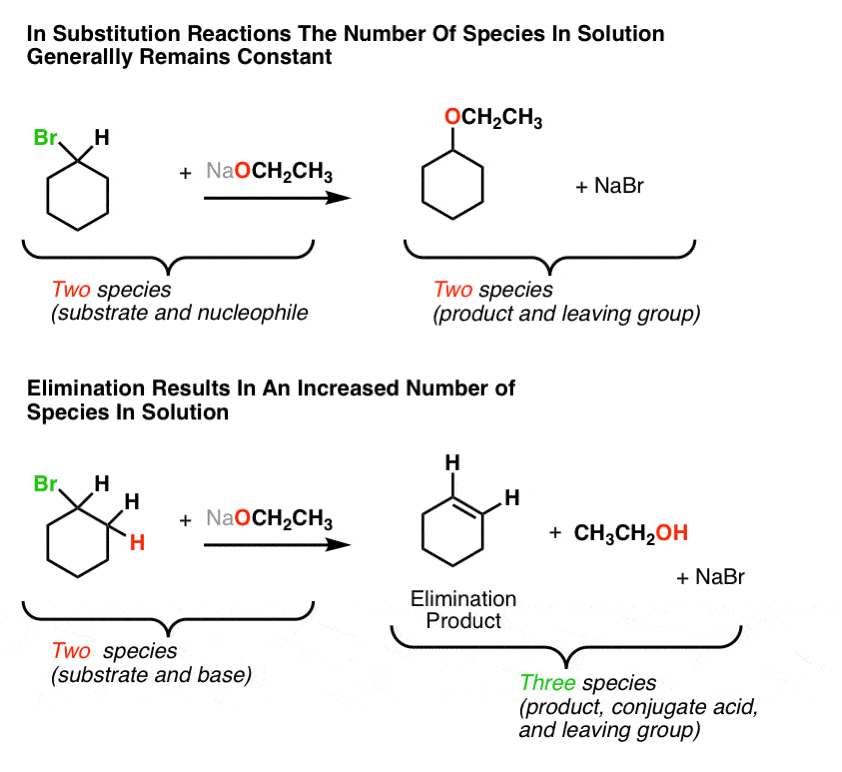


Elimination Reactions Are Favored By Heat Master Organic Chemistry



Gibbs Energy


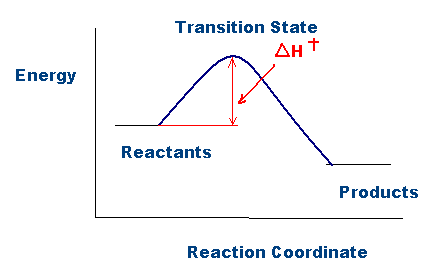
Transition State Theory Wikipedia



Entropy In Chemistry Definition Law Chemistry Class Video Study Com



Write A Relation Between Delta G And Q And Define The Meaning Of Each Term And Answer The Following A Why A Reaction Proceeds Forward When Q K And No Chemistry


Gibbs Free Energy



Chem 180 Set 1 Flashcards Quizlet


Thermodynamics I Chemistry Revision Site


Electrolytic Cells An Electrochemical Cell In Which The Chemistry Is Non Spontaneous Is Called A Electrolytic Cell This Means That The Oxidation Will Not Occur Spontaneously At The Anode And The Reduction Will Not Be Spontaneous At The Cathode The Chemistry
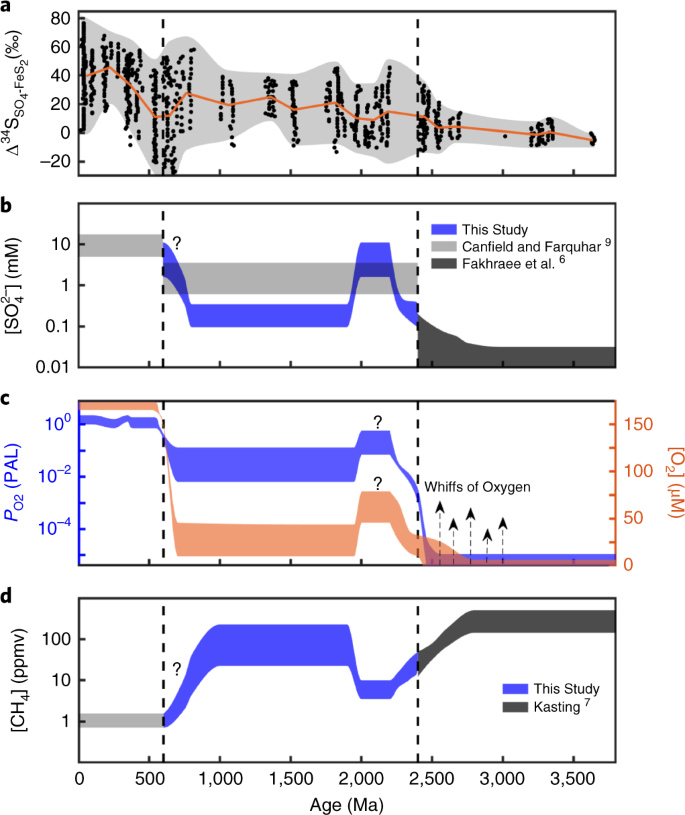


Proterozoic Seawater Sulfate Scarcity And The Evolution Of Ocean Atmosphere Chemistry Nature Geoscience


Polarity



Hess S Law Definition Formula Examples Video Lesson Transcript Study Com



Gibbs Free Energy Dg Dh Tds Chad S Prep



Chemical Thermodynamics Definition Principles Video Lesson Transcript Study Com


Chemical Thermodynamics



Gibbs Free Energy Boundless Chemistry



What Is Delta 8 Tetrahydrocannabinol Delta 8 Tetrahydrocannabinol Definition By Weedmaps
:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)


Endergonic Vs Exergonic Reactions And Processes



Delta Scientific Hydrogen Is The Lightest Element In The Periodic Table And The Most Plentiful In The Universe View More Interesting Facts About Hydrogen Iypt19 Periodictable Science



Is It A Spontaneous Reaction Delta G Tells You Youtube



Making Sense Of G And G When It Comes To Equilibrium Adrian Dingle S Chemistry Pages



Chemistry Tutoring Explaining Greek Symbols In Organic Chemistry



Spontaneous Reaction Definition Examples Video Lesson Transcript Study Com
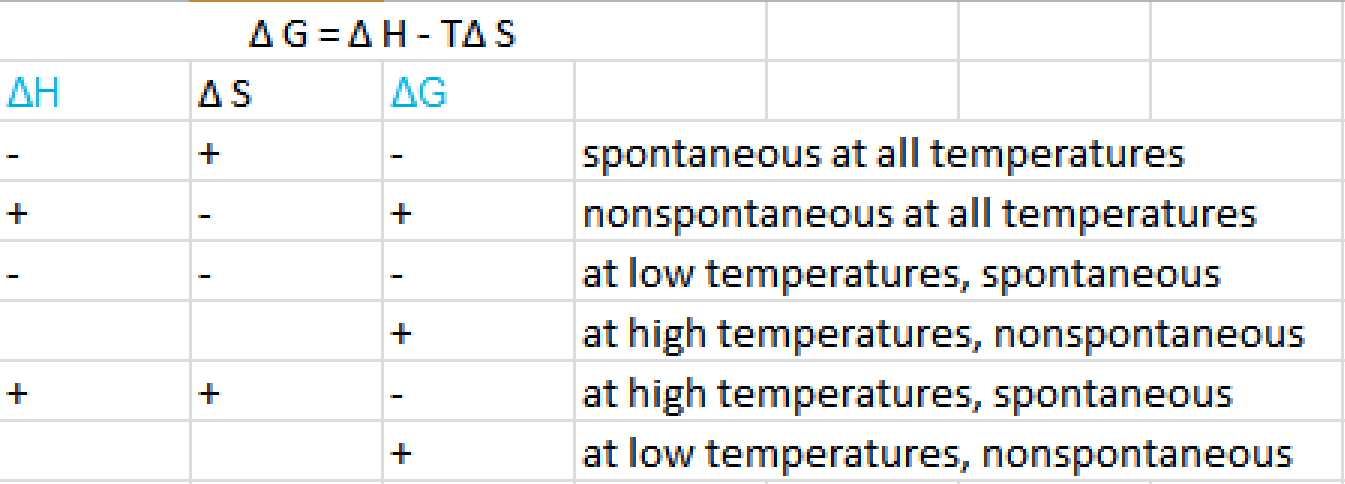


What Is Delta G Socratic



What Does Delta S Stand For In Chemistry Quora



19 7 Dg And K As Functions Of Temperature Chemistry Libretexts



Chemistry The Central Science Chapter 5 Section 7
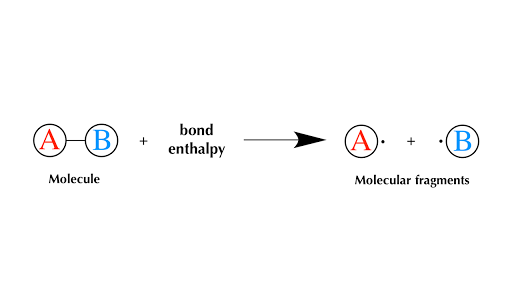


Bond Enthalpy And Enthalpy Of Reaction Article Khan Academy



Heat Of Fusion Heat Of Vaporization Concept Chemistry Video By Brightstorm



Gibbs Free Energy


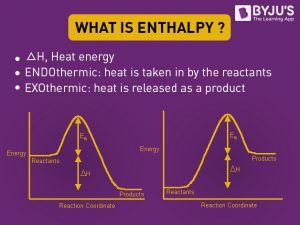
Enthalpy Definition Enthalpy Units And What Is Thermodynamics Byju S
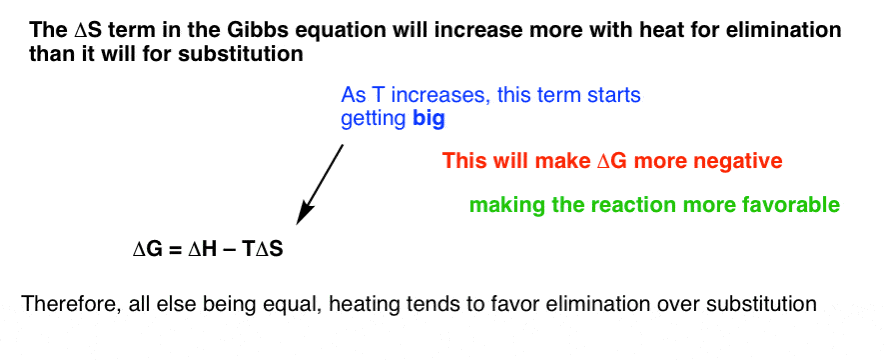


Elimination Reactions Are Favored By Heat Master Organic Chemistry
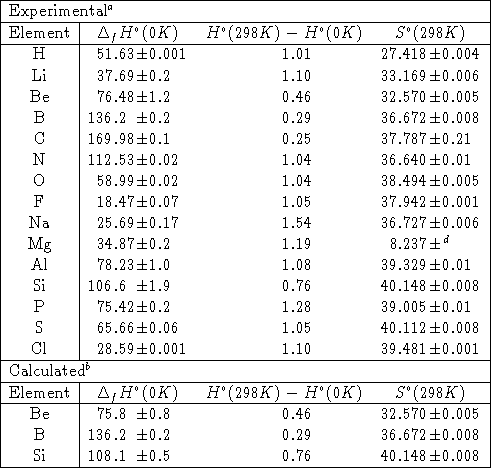


Thermochemistry In Gaussian Gaussian Com



3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
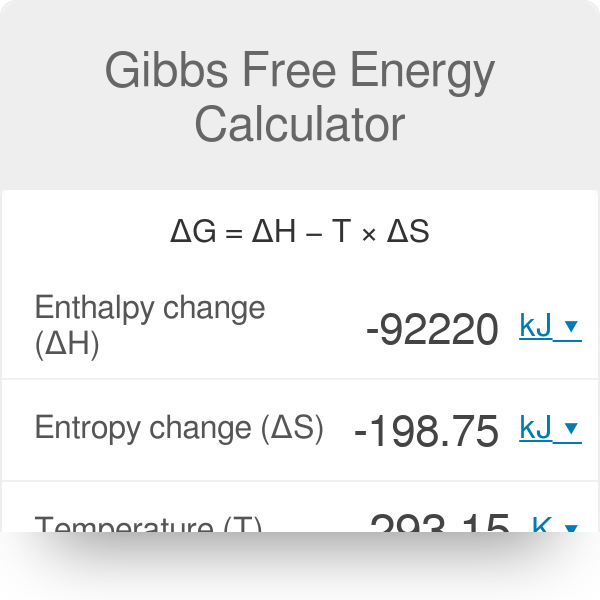


Gibbs Free Energy Calculator
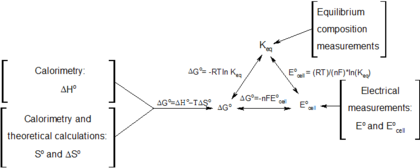


Gibbs Free Energy Wikipedia
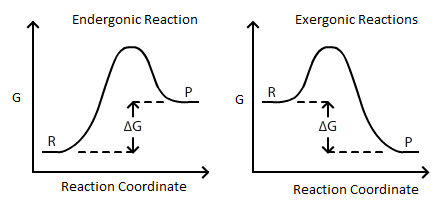


Connection Between E Cell G And K Chemistry Libretexts



The Relationship Between Free Energy And The Equilibrium Constant Science Class Video Study Com


Delta National Geographic Society
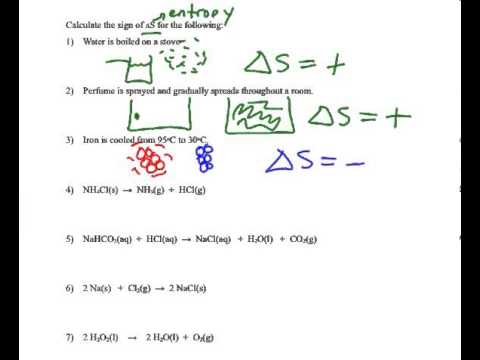


Determining The Sign Of The Entropy Change Delta S Youtube



Chemistry 6 7 Delta G Delta H Delta S Flashcards Quizlet



River Deltas Introduction Formation Types Questions Toppr



Thermite Reaction Aluminum Reacts With Iron Iii Oxide Chemdemos
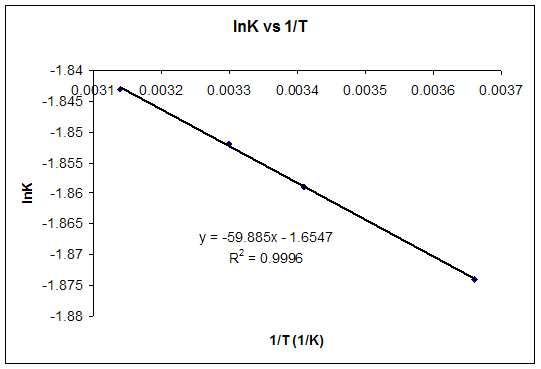


Gibbs Free Energy
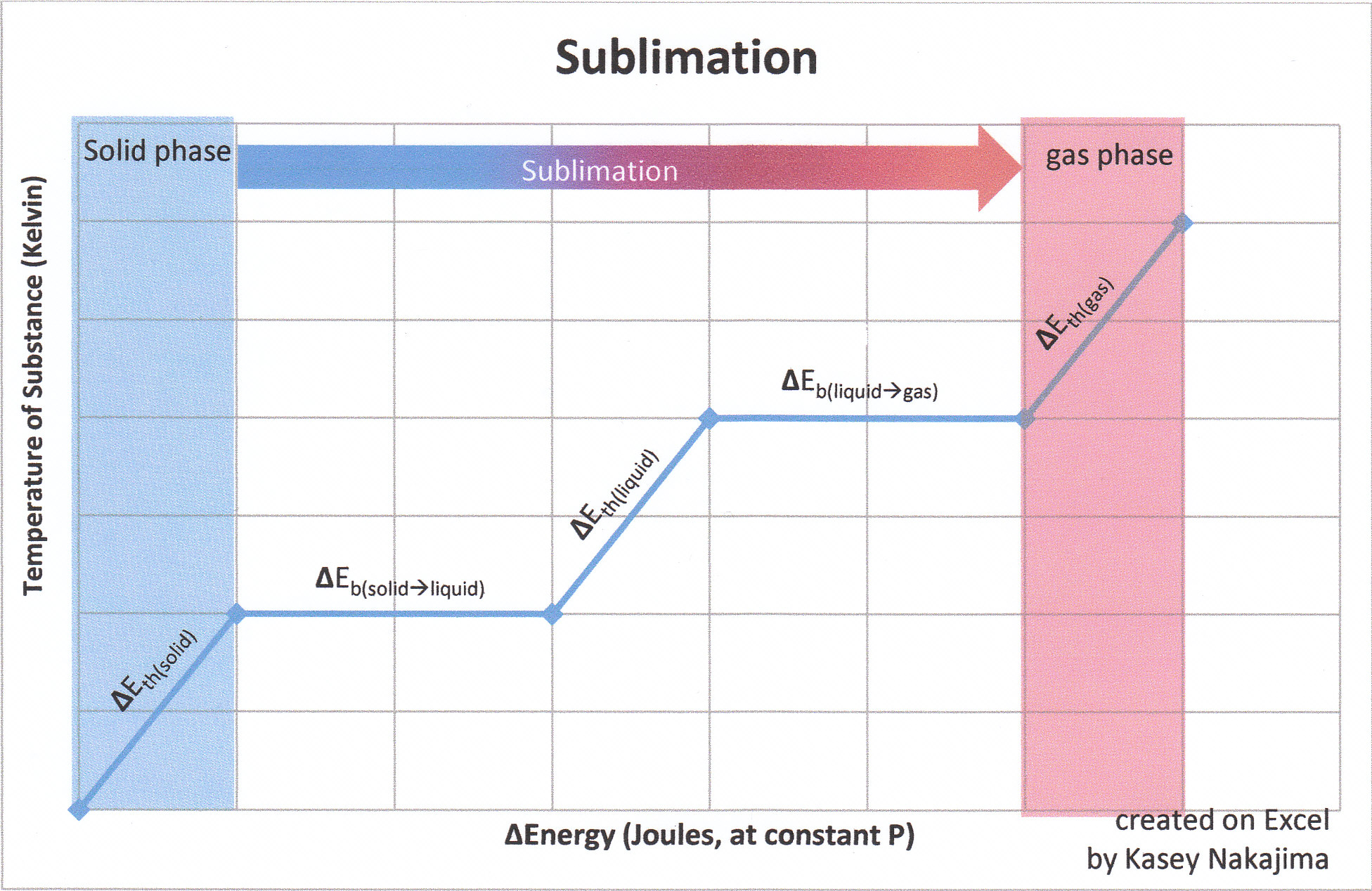


Heat Of Sublimation Chemistry Libretexts



Solved From The Following Rate Constants Determi



Meaning Of Delta I H Here Chemistry Meritnation Com



0 件のコメント:
コメントを投稿